

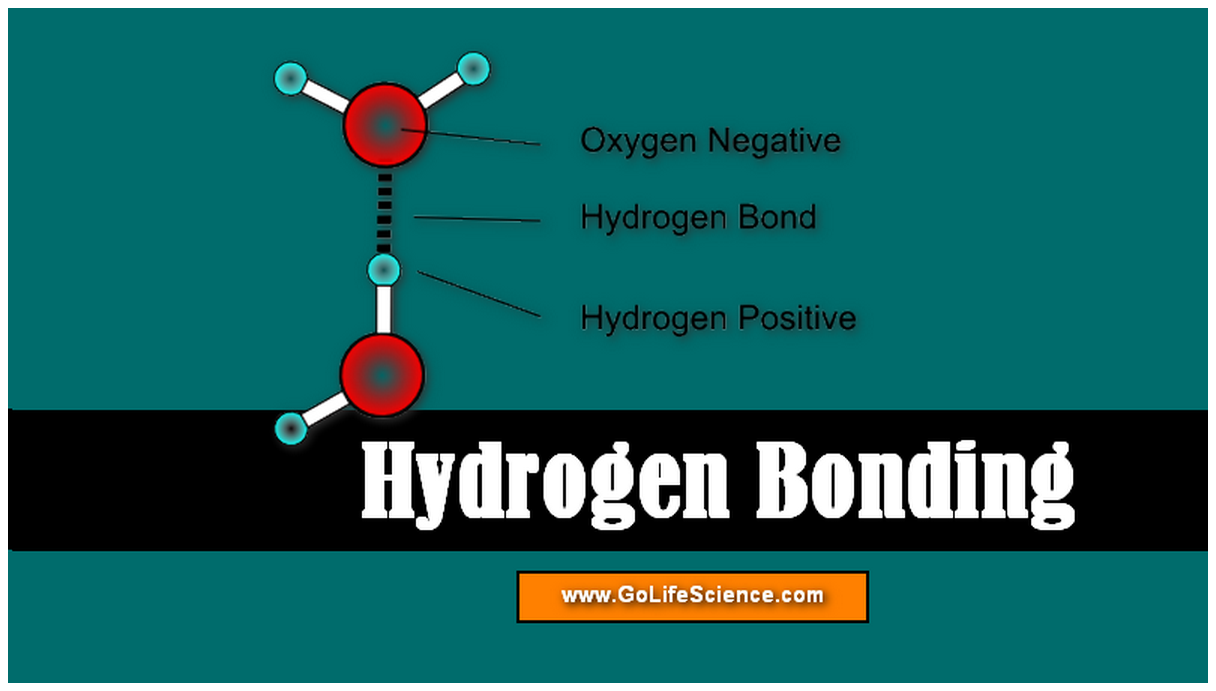
The atom that gains an electron becomes a negative ion. The atom that loses an electron becomes a positive ion. Obviously the hydrogen and fluorine atoms in adjacent molecules are not just “touching,” but must be associated in a much more intimate way.Ionic bonds form when one atom transfers electrons to another atom. If we add the van der Waals radii for hydrogen and fluorine, we obtain an expected hydrogen to fluorine distance of (120 + 135) pm = 255 pm, over 100 pm larger than that observed. Here the distance between hydrogen and fluorine nuclei in different molecules is only 150 pm. Hydrogen bonding between HF molecules is particularly evident in solid HF, where the atoms are arranged in a zigzag pattern: Thus hydrogen bonding can account for the unusually high boiling points of NH 3, H 2O, and HF. Separation of two molecules joined by a hydrogen bond requires 10 to 30 kJ mol –1, roughly 10 times the energy needed to overcome dipole forces.
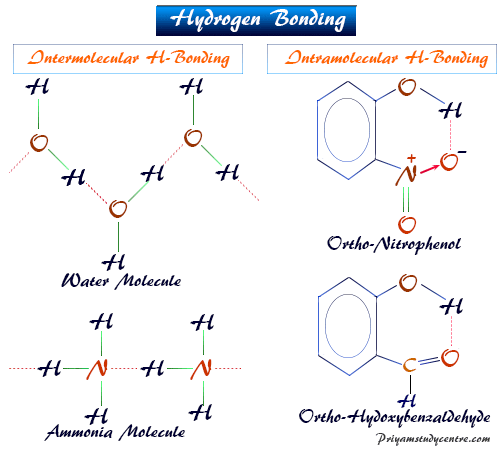
The other molecule must have a very electronegative atom (again usually fluorine, oxygen, or nitrogen) which has one or more lone pairs of electrons. In order for hydrogen bonding to occur, there must be a hydrogen atom connected to a small, highly electronegative atom (usually fluorine, oxygen, or nitrogen) in one molecule. The close approach of oppositely charged ends of molecular dipoles combines with this small degree of covalent-bond character to produce an abnormally strong intermolecular force called a hydrogen bond. Some covalent-bond character develops between fluorine in one molecule and hydrogen in the other.

The attractive force between the electrons of the left-hand fluorine and the right-hand hydrogen nucleus will be unusually strong, and there will be considerable distortion of the electron clouds. Also, because fluorine is such an electronegative atom, its partial negative charge is particularly large. The sphere on the right is oriented the same way with the positive side attracted to the "F" side of the first sphere.Ĭonsequently, the hydrogen nucleus in the HF on the right can approach much closer to the fluorine side of the left-hand molecule than could any other kind of nucleus. Most of the sphere corresponds to cloud of "F". The left sphere has a bulge on its left side corresponding to "H" and a partially positive charge. In each HF molecule, the hydrogen nucleus is rather poorly shielded by a thin electron cloud (only two electrons), and much of that electron cloud has been distorted toward the highly electronegative fluorine atom.ĭiagram of two distorted spheres. Suppose that two HF molecules approach each other, as shown in the following figure. In order to see why this happens, let us consider the simplest second-row hydride-HF. Clearly these second-row hydrides must have particularly strong intermolecular forces. In the second period, however, the polar hydrides NH 3, H 2O, and HF all have boiling points more than 100☌ above that of the nonpolar compound CH 4. Similar behavior occurs among the hydrides of elements in the fourth and third periods. SbH 3, H 2Te and HI, all of which are polar, have somewhat higher boiling points, but all lie within a range of 50☌.
SnH 4, which consists of nonpolar molecules, boils at the lowest temperature. Hydrides of elements in the fifth period behave as we might predict. Note the anomalously high boiling points of H2O, HF, and NH3 in the second period. \) The boiling points of the hydrides of the nonmetals plotted against the period in which they occur in the periodic table.


 0 kommentar(er)
0 kommentar(er)
